ASAP PLS
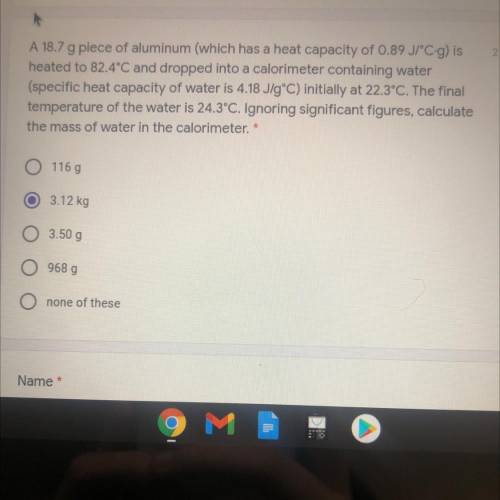
A 18.7 g piece of aluminum (which has a heat capacity of 0.89 JPC-g) is
heated to 82...

ASAP PLS
A 18.7 g piece of aluminum (which has a heat capacity of 0.89 JPC-g) is
heated to 82.4°C and dropped into a calorimeter containing water
(specific heat capacity of water is 4.18 J/gºC) initially at 22.3°C. The final
temperature of the water is 24.3°C. Ignoring significant figures, calculate
the mass of water in the calorimeter. *

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1


Chemistry, 23.06.2019 00:10, graceception
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 31.08.2020 17:01

Mathematics, 31.08.2020 17:01

Mathematics, 31.08.2020 17:01


Mathematics, 31.08.2020 17:01

Social Studies, 31.08.2020 17:01

Mathematics, 31.08.2020 17:01

Mathematics, 31.08.2020 17:01

Physics, 31.08.2020 17:01

English, 31.08.2020 17:01



