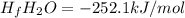A scientist measures the standard enthalpy change for the following reaction to be 67.9 kJ:
Fe2O3(s) + 3 H2(
92Fe(s) + 3 H2O(9)
Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation
of H2O(g) is
kJ/mol.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1


Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3
You know the right answer?
A scientist measures the standard enthalpy change for the following reaction to be 67.9 kJ:
Fe2O3(s...
Questions in other subjects:


English, 30.07.2019 06:00

Business, 30.07.2019 06:00

Social Studies, 30.07.2019 06:00



Computers and Technology, 30.07.2019 06:00


Biology, 30.07.2019 06:00

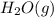 is -252.1 kJ/mol.
is -252.1 kJ/mol.
![\Delta H=[n\times H_f_{products}]-[n\times H_f_{reactantss}]](/tpl/images/1252/1104/6418d.png)
![\Delta H=[2\times H_f{Fe}+3\times H_f{H_2O}]-[1\times H_f{Fe_2O_3}+3\times H_f{H_2}]](/tpl/images/1252/1104/83e3e.png)
![67.9kJ=[(2\times 0)+(3\times H_f{H_2O})]-[(1\times -824.2kJ/mol)+3\times 0kJ/mol)]](/tpl/images/1252/1104/4a92c.png)