
Chemistry, 12.04.2021 08:20 shadoris26
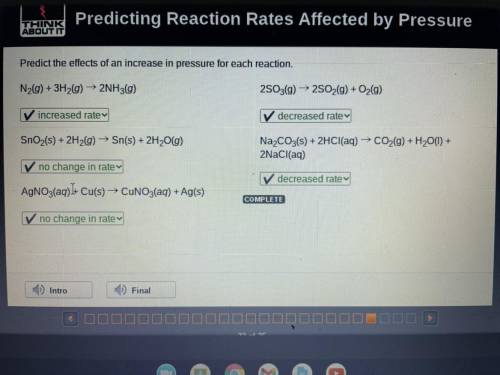
Predict the effects of an increase in pressure for each reaction.
N2(g) + 3H2(g) → 2NH3(g) 2S03(g) ► 2S02(g) + O2(g)
SnO2(s) + 2H2(g) → Sn(s) + 2H20(g)
Na2CO3(s) + 2HCl(aq) → CO2(g) + H20(1) +
2NaCl(aq)
AgNO3(aq))+ Cu(s) → CUNO3(aq) + Ag(s)
COMPLETE

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 14:50, rebeccamckellpidge
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
Predict the effects of an increase in pressure for each reaction.
N2(g) + 3H2(g) → 2NH3(g) 2S03(g)...
Questions in other subjects:

Mathematics, 28.08.2019 07:00

Physics, 28.08.2019 07:00

Chemistry, 28.08.2019 07:00


History, 28.08.2019 07:10

English, 28.08.2019 07:10

Health, 28.08.2019 07:10

Biology, 28.08.2019 07:10

English, 28.08.2019 07:10

Mathematics, 28.08.2019 07:10



