One molecule of a compound weighs 2.93 10–22 g. The molar mass of this compound is:
A)
2.06...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, colochaortiz20p7cajw
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3


Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 27.04.2021 03:00

Social Studies, 27.04.2021 03:00


Mathematics, 27.04.2021 03:00

Mathematics, 27.04.2021 03:00


Health, 27.04.2021 03:00

Mathematics, 27.04.2021 03:00

Mathematics, 27.04.2021 03:00

Arts, 27.04.2021 03:00

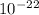 ) × (6.022×
) × (6.022×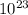 ) = 176.44 g/mol
) = 176.44 g/mol

