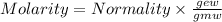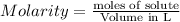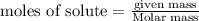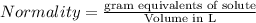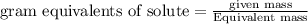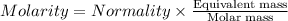Chemistry, 11.04.2021 14:00 joelpimentel
The relation between molarity and normality is expresses as: a) M = N x g. e.w/g. m.w
b) M = N x g. m.w/ g. e.w
c) M = N x no. of equiv./mole
d) M = N x no. of g. m.w/mole

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, black99girl
What type of reaction is represented by the following example? 2co2 (g) + 4h2o (l) + 1452 kj 2ch3oh (l) (g) + 3o2 (g) exothermic endothermic
Answers: 1

Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1
You know the right answer?
The relation between molarity and normality is expresses as: a) M = N x g. e.w/g. m.w
b) M = N x g....
Questions in other subjects: