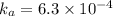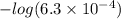Chemistry, 11.04.2021 08:20 Teedollasign
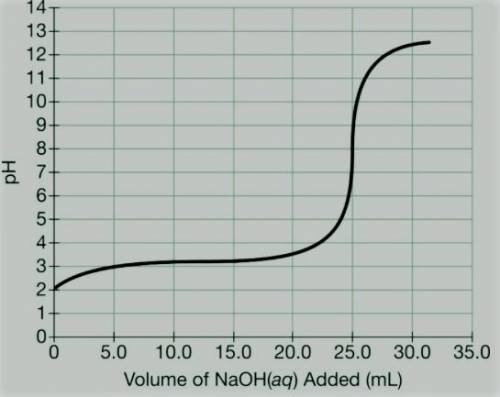
c) A student titrated 50.0mL of a 0.10M solution of a certain weak acid with NaOH(aq) . The results are given in the graph above. (i) What is the approximate pKa of the acid?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
c) A student titrated 50.0mL of a 0.10M solution of a certain weak acid with NaOH(aq) . The results...
Questions in other subjects:


SAT, 30.11.2021 23:10

History, 30.11.2021 23:10


SAT, 30.11.2021 23:10



SAT, 30.11.2021 23:10


![[H^+] = 10^{-2.1}](/tpl/images/1251/1956/a82c7.png)
![[H^+] = ({k_a \times 0.10})^{\dfrac{1}{2}}](/tpl/images/1251/1956/45c21.png)