Question 3:
How many atoms are in 25.00 g of B.
A. 1.393 x 10^24 atoms of B
B. 1.333 x...

Chemistry, 10.04.2021 09:10 etxchrissy
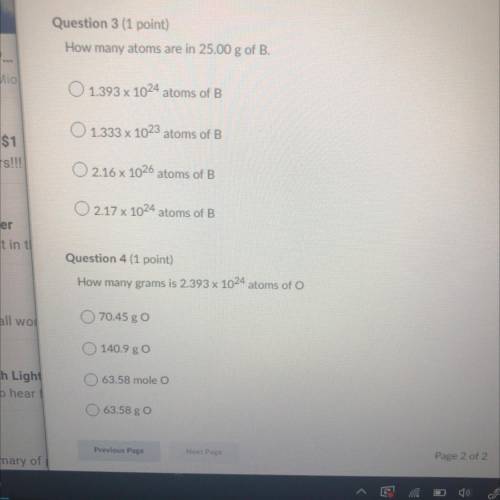
Question 3:
How many atoms are in 25.00 g of B.
A. 1.393 x 10^24 atoms of B
B. 1.333 x 10^23 atoms of B
C. 2.16 x 10^26 atoms of B
D.217 x 10^24 atoms of B
Question 4:
How many grams is 2.393 x 10^24 atoms of O
A. 70.45 g O
B. 140.9 g O
C. 63.58 mole O
D. 63.58 g O
Please answer both questions if you can, thanks for all the efforts :)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, zionlopez543
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1



Chemistry, 23.06.2019 05:00, jayden6467
How many moles are in 7.2 x 10^23 carbon molecules?
Answers: 1
You know the right answer?
Questions in other subjects:

English, 14.11.2019 06:31


Mathematics, 14.11.2019 06:31




French, 14.11.2019 06:31

English, 14.11.2019 06:31


Chemistry, 14.11.2019 06:31



