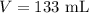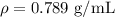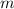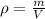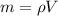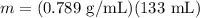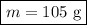Chemistry, 10.04.2021 06:50 jagslovegirl
Ethanol is a common laboratory solvent and has a density of 0.789 g/mL. What is the mass, in grams, of 133 mL of ethanol?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 23:00, 1315055427
Which subshell is represented by the actinides family?
Answers: 1

Chemistry, 23.06.2019 03:00, duplessistoccara
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2
You know the right answer?
Ethanol is a common laboratory solvent and has a density of 0.789 g/mL. What is the mass, in grams,...
Questions in other subjects:




Mathematics, 14.05.2021 20:00

Arts, 14.05.2021 20:00

Mathematics, 14.05.2021 20:00


Chemistry, 14.05.2021 20:00