
Chemistry, 10.04.2021 01:10 mendezmarco2004
A 112g sample of CO(g) is combined with a 32g sample of O2(g) and the reaction represented proceeds as completely as possible. Which TWO of the following statements are correct? *
A. CO(g) is the limiting reactant.
B. O2(g) is the limiting reactant.
C. 4mol of CO(g) reacts.
D. 2mol of CO(g) remains unreacted.
E. 144g of CO2(g) is produced.
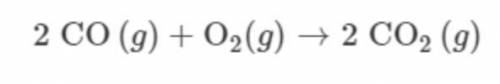

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 20:30, trevorhenyan51
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
A 112g sample of CO(g) is combined with a 32g sample of O2(g) and the reaction represented proceeds...
Questions in other subjects:


Mathematics, 12.02.2021 18:30



Mathematics, 12.02.2021 18:30

Mathematics, 12.02.2021 18:30







