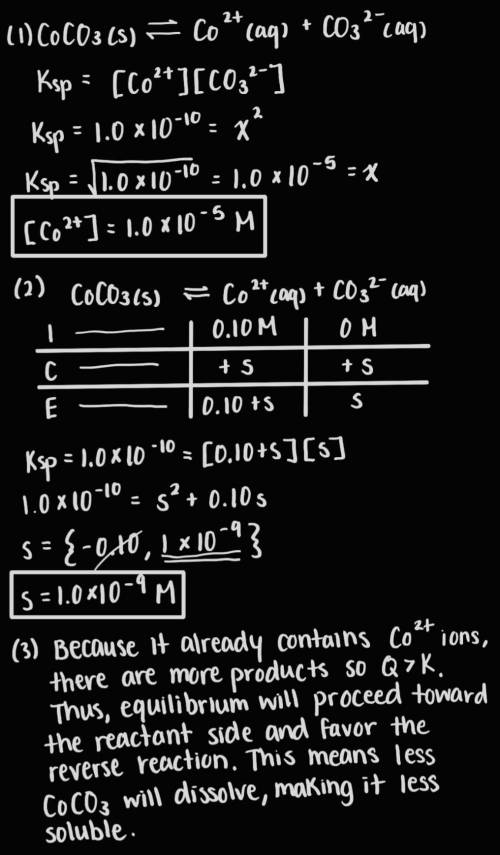Chemistry, 09.04.2021 23:50 valoiserika1229
Answer the following questions about the solubility of CoCO3(s). The value of Ksp for CoCO3(s) is 1.0 × 10^−10.
A. Calculate the value of [Co2+] in a saturated solution of CoCO3 in distilled water.
B. If 0.10 M of Co2+ is already present in distilled water, calculate the molar solubility of CoCO3(s).
C. Explain why CoCO3 is less soluble in distilled water that already contains Co2+

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 18:00, brisacruz013
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 20:00, emilyswinge4421
Listenbase your answer to the question on the information below. nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body. cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment. which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

Chemistry, 23.06.2019 03:30, LlayahHarbin
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
Answer the following questions about the solubility of CoCO3(s). The value of Ksp for CoCO3(s) is 1....
Questions in other subjects:

Mathematics, 07.04.2020 21:58

Biology, 07.04.2020 21:58








![K = \frac{[products]}{[reactants]}](/tpl/images/1250/0658/0c10f.png) increases.
increases.