
Chemistry, 09.04.2021 17:00 jorgefrom584
When copper metal is affed to silver nitrate in solution, silver metal and copper (II) nitrate are produced. Use the balanced equation to answer the question. How many grams of silver are produced from 4.00 moles of copper?
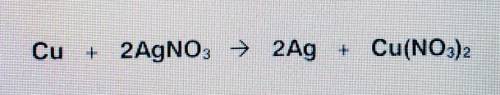

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
When copper metal is affed to silver nitrate in solution, silver metal and copper (II) nitrate are p...
Questions in other subjects:



Geography, 27.09.2019 04:30

History, 27.09.2019 04:30




Physics, 27.09.2019 04:30

Biology, 27.09.2019 04:30

Mathematics, 27.09.2019 04:30



