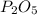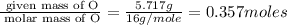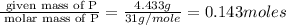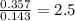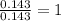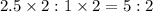Chemistry, 09.04.2021 09:20 harris435942
A 10.150g sample of a compound known to contain phosphorus and oxygen yields 5.717 g of oxygen. Find the empirical formula of the compound

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, maevemboucher78
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 14:30, Priskittles
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 23.06.2019 00:00, tonimgreen17p6vqjq
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2
You know the right answer?
A 10.150g sample of a compound known to contain phosphorus and oxygen yields 5.717 g of oxygen. Find...
Questions in other subjects:



Mathematics, 04.11.2019 01:31


English, 04.11.2019 01:31



Social Studies, 04.11.2019 01:31

English, 04.11.2019 01:31

Mathematics, 04.11.2019 01:31