
Chemistry, 08.04.2021 22:20 cornpops4037
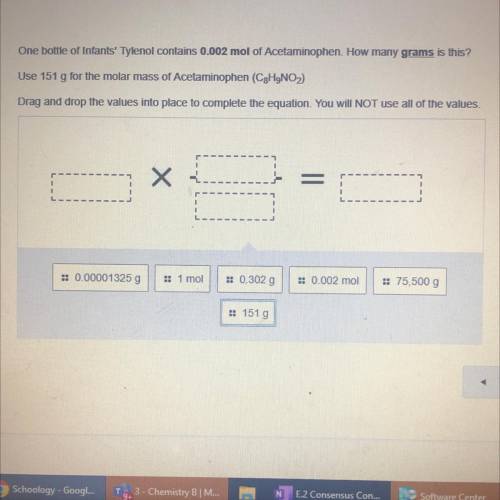
One bottle of Infants' Tylenol contains 0.002 mol of Acetaminophen. How many grams is this?
Use 151 g for the molar mass of Acetaminophen (C2H9NO)
Drag and drop the values into place to complete the equation. You will NOT use all of the values.
Х
= 0.00001325 g
1 moi
:: 0.302 g
: 0.002 mol
= 75,500 g
:: 151 g

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 23.06.2019 03:30, Ramann03
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3
You know the right answer?
One bottle of Infants' Tylenol contains 0.002 mol of Acetaminophen. How many grams is this?
Use 151...
Questions in other subjects:

Physics, 19.07.2019 11:00

Mathematics, 19.07.2019 11:00


Mathematics, 19.07.2019 11:00

Biology, 19.07.2019 11:00


English, 19.07.2019 11:00

Mathematics, 19.07.2019 11:00


Mathematics, 19.07.2019 11:00



