
Chemistry, 08.04.2021 01:40 Raechelrae04
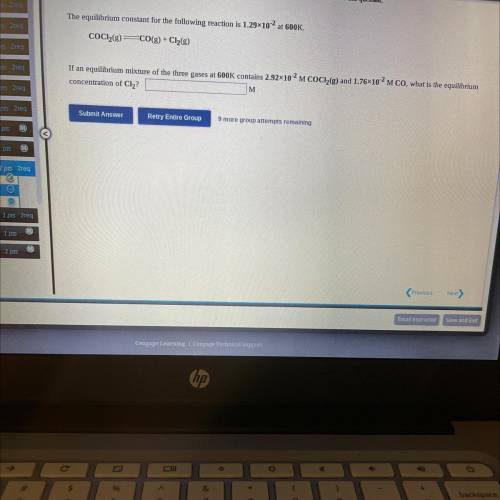
If an equilibrium mixture of the three gases at 600K contains 2.92*10^-2 M COCH(g) and 1.76*10^2 M CO, what is the equilibrium concentration of Cl2?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:30, bibiansolis
The table lists the lattice energies of some compounds. compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf. the lattice energy increases as the cations get larger, as shown by lif and licl. the lattice energy decreases as cations get smaller, as shown by nacl and naf. the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3


Chemistry, 23.06.2019 01:00, aliviadushane
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
You know the right answer?
If an equilibrium mixture of the three gases at 600K contains 2.92*10^-2 M COCH(g) and 1.76*10^2 M C...
Questions in other subjects:

Mathematics, 27.03.2020 21:32


Geography, 27.03.2020 21:32

History, 27.03.2020 21:32

Mathematics, 27.03.2020 21:32

Mathematics, 27.03.2020 21:32



Mathematics, 27.03.2020 21:32




