What effect would an increase in pressure have on this reversible reaction at Equilibrium
...

Chemistry, 07.04.2021 20:20 chessacs6540
What effect would an increase in pressure have on this reversible reaction at Equilibrium
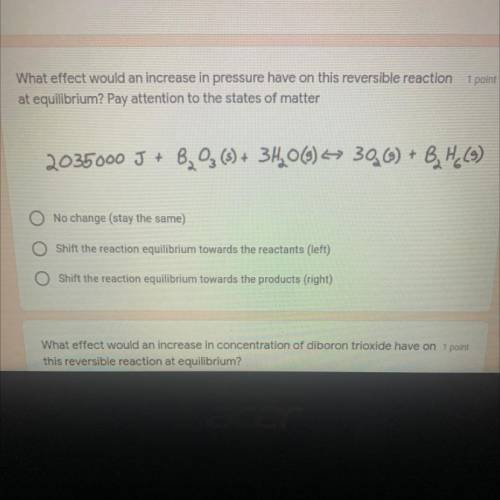

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
Questions in other subjects:


Biology, 03.12.2020 01:50

Mathematics, 03.12.2020 01:50

History, 03.12.2020 01:50

Physics, 03.12.2020 01:50

Health, 03.12.2020 01:50


Computers and Technology, 03.12.2020 01:50


Spanish, 03.12.2020 01:50



