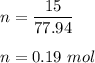What is the number of moles in 15.0 g AsH3?
O 5.2 mol
O 1200 mol
O 0.44 mol
O 0.1...

Chemistry, 07.04.2021 06:20 andrewjarrah05
What is the number of moles in 15.0 g AsH3?
O 5.2 mol
O 1200 mol
O 0.44 mol
O 0.19 mol

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 27.01.2021 14:00

History, 27.01.2021 14:00

Mathematics, 27.01.2021 14:00


English, 27.01.2021 14:00


Mathematics, 27.01.2021 14:00

English, 27.01.2021 14:00

Social Studies, 27.01.2021 14:00

Mathematics, 27.01.2021 14:00