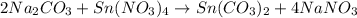Chemistry, 06.04.2021 06:50 arrissa1234hinkle
A double-replacement reaction takes place when aqueous Na, co, reacts with aqueous Sn(NO3), according to the following equation. Na, co, + Sn(NO,)
You would expect one of the products of this reaction to be
20
Sn2(CO3)2
NaNO,
O Nas
OCNO,

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1
You know the right answer?
A double-replacement reaction takes place when aqueous Na, co, reacts with aqueous Sn(NO3), accordin...
Questions in other subjects:







English, 25.11.2021 05:40

Social Studies, 25.11.2021 05:40

Mathematics, 25.11.2021 05:40

Social Studies, 25.11.2021 05:40

 and
and  .
.