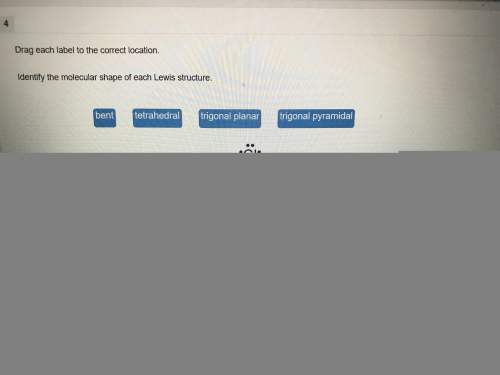
Chemistry, 06.04.2021 01:50 heyperdomo4369
For each pair of covalently bonded atoms, choose the one expected to have the shortest bond length. (A) C-I (B) H-I (A, B) fill in the blank 1 (C) H-Cl (D) H-I (C, D) fill in the blank 2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2


You know the right answer?
For each pair of covalently bonded atoms, choose the one expected to have the shortest bond length....
Questions in other subjects:

Chemistry, 18.11.2019 04:31

Mathematics, 18.11.2019 04:31



Mathematics, 18.11.2019 04:31

History, 18.11.2019 04:31

Social Studies, 18.11.2019 04:31

Geography, 18.11.2019 04:31

Biology, 18.11.2019 04:31

English, 18.11.2019 04:31