
Chemistry, 05.04.2021 19:40 softballgirl3589
6. Determine the molar mass of an unknown gas that has a volume of 72.5 mL at a temperature of
68.0°C, and a pressure of 0.980 atm, and a mass of 0.207 g.
(hint: find moles first and remember that molar mass is the mass per mole”)
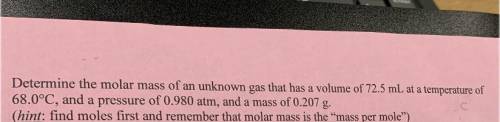

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, hardwick744
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2


Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 05:00, rosezgomez97
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1
You know the right answer?
6. Determine the molar mass of an unknown gas that has a volume of 72.5 mL at a temperature of
68.0...
Questions in other subjects:

Computers and Technology, 30.06.2021 14:00

Social Studies, 30.06.2021 14:00



English, 30.06.2021 14:00



Mathematics, 30.06.2021 14:00


Mathematics, 30.06.2021 14:00



