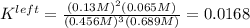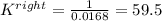Chemistry, 05.04.2021 03:50 addisynshepherd
2A (g) + Y (g) <-- --> 3C (g) + D (g)
Based on the initial conditions shown below, determine the value of the equilibrium constant if the concentration of product C at equilibrium was measured to be 0.456 M.
Initial Conditions: No reactants present, [C] = 0.651 M, [D] = 0.754 M.
Equilibrium Conditions: [A] = ?, [Y] = ?, [C] = 0.456 M, [D] = ?.
A. K = 59.5
B. K = 37.2
C. K = 0.0269
D. K = 0.0168

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 22:30, gonzalesalexiaouv1bg
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
2A (g) + Y (g) <-- --> 3C (g) + D (g)
Based on the initial conditions shown below, determine...
Questions in other subjects:

History, 05.07.2019 16:30

Mathematics, 05.07.2019 16:30

Biology, 05.07.2019 16:30

Mathematics, 05.07.2019 16:30

History, 05.07.2019 16:30

Biology, 05.07.2019 16:30


Chemistry, 05.07.2019 16:30


![K^{left}=\frac{[A]^2[Y]}{[C]^3[D]}](/tpl/images/1239/1292/6d673.png)
![[A]=2x](/tpl/images/1239/1292/d41d8.png)
![[Y]=x](/tpl/images/1239/1292/d76b1.png)
![[C]=0.651M-3x](/tpl/images/1239/1292/474d4.png)
![[D]=0.754M-x](/tpl/images/1239/1292/68d18.png)
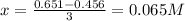
![[A]=2(0.065M)=0.13M](/tpl/images/1239/1292/b6025.png)
![[Y]=0.065M](/tpl/images/1239/1292/afc74.png)
![[D]=0.754M-0.065M=0.689M](/tpl/images/1239/1292/0e883.png)