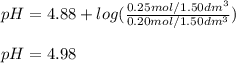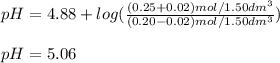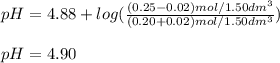A buffer solution contains 0.20 mol of propionic acid (CH3CH2COOH) and 0.25 mol of sodium propionate (CH3CH2COONa) in 1.50 dm3.
What is the pH of this buffer?
Enter your answer using two decimal places.
What is the pH of the buffer after the addition of 0.02 mol of NaOH?
What is the pH of the buffer after the addition of 0.02 mol of HI?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1

Chemistry, 22.06.2019 20:00, rafaelasoareschagas7
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3


Chemistry, 22.06.2019 23:00, liv467
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
A buffer solution contains 0.20 mol of propionic acid (CH3CH2COOH) and 0.25 mol of sodium propionate...
Questions in other subjects:

SAT, 28.12.2021 16:30



Business, 28.12.2021 16:40

Business, 28.12.2021 16:40





![pH=pKa+log(\frac{[base]}{[acid]} )](/tpl/images/1238/6169/33848.png)