
Chemistry, 03.04.2021 05:40 nadiareese
how much alum product would be lost to the crystallization solution if you had 42.5 ml of solution after filtration and the solubility of alum is approximately 2.63 g alum in 100 ml of 0 degrees celcius acidic water

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1


Chemistry, 23.06.2019 01:40, mandilynn22
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
how much alum product would be lost to the crystallization solution if you had 42.5 ml of solution a...
Questions in other subjects:










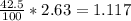 grams
grams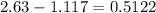 grams
grams 

