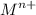Chemistry, 03.04.2021 04:40 amandasantiago2001
Sally has constructed a concentration cell to measure Ksp for MCln. She constructs the cell by adding 2 mL of 0.05 M M(NO3)n to one compartment of the microwell plate. She then makes a solution of MCln by adding KCl to M(NO3)n. She adds 6.380 mL of the resulting mixture to a second compartment of the microwell plate.
Sally knows n (the charge on the metal ion) = +2
She has already calculated [Mn+] in the prepared MCln solution using the Nernst equation. [Mn+] = 8.279 M
Required:
How many moles of [Cl-] must be dissolved in that compartment?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 23:00, soccerplayer17
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2
You know the right answer?
Sally has constructed a concentration cell to measure Ksp for MCln. She constructs the cell by addin...
Questions in other subjects:




Mathematics, 09.06.2020 02:57

Mathematics, 09.06.2020 02:57

Mathematics, 09.06.2020 02:57




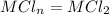
![$[M^{n+}]=[m^{2+}]=8.279 \ M$](/tpl/images/1238/0273/0e0ea.png)
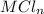 taken in that compartment = 6.380 mL
taken in that compartment = 6.380 mL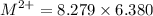
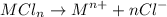
 = 2 x moles of
= 2 x moles of