
Chemistry, 02.04.2021 18:30 lovelarissa
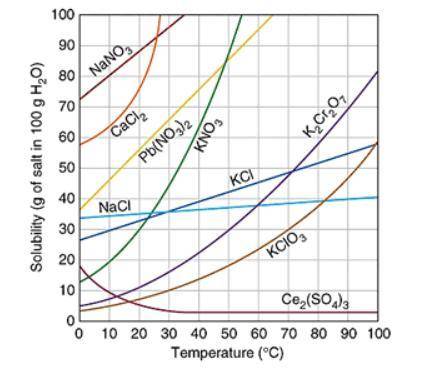
Assuming that the trends continue, which of the following compound will have the greatest solubility at 120 ℃? The graph below shows the solubility of a variety of compounds.
A. Ce2(SO4)3
B. K2Cr2O7
C. NaCl
D. Pb(NO3)2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2


Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

You know the right answer?
Assuming that the trends continue, which of the following compound will have the greatest solubility...
Questions in other subjects:


Chemistry, 06.11.2019 02:31

Business, 06.11.2019 02:31







Mathematics, 06.11.2019 02:31



