
Chemistry, 02.04.2021 08:10 smithmorgan773p35885
If 10.00 moles of copper are reacted with 6.00 moles of sulfur according to the following balanced equation, which reactant is the limiter and how many moles of excess reactant would remain after the reaction is completed? 2 Cu + S --> Cu2S
Cu is limiting and 1.00 mole of excess S remain
S is limiting and 1.00 moles of excess Cu remain
S is limiting and 4.00 moles of excess Cu remain
Cu is limiting and 4.00 moles of excess S remain

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, ronny80
Supongamos que estás estudiando dos estrellas. ambas estrellas tienen la misma magnitud aparente, pero la estrella a tiene una magnitud absoluta mayor que la estrella b. ¿que puedes decir acerca de la distancia a la tierra de estas dos estrellas?
Answers: 3



Chemistry, 23.06.2019 06:30, OsoDeOro7968
Which of these natural resources is non-renewable a. corn b. wind c. geothermal d. natural gas
Answers: 2
You know the right answer?
If 10.00 moles of copper are reacted with 6.00 moles of sulfur according to the following balanced e...
Questions in other subjects:

Health, 02.11.2019 17:31

Mathematics, 02.11.2019 17:31

History, 02.11.2019 17:31



History, 02.11.2019 17:31



History, 02.11.2019 17:31

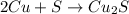
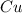 require 1 mole of
require 1 mole of 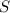
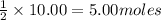 of
of 

