
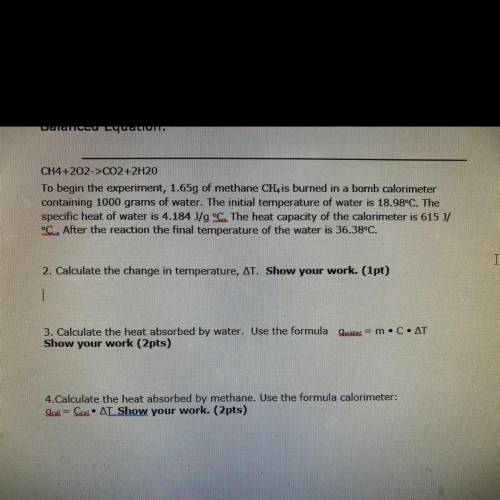
CH4+202->CO2+2H20
To begin the experiment, 1.65g of methane CH is burned in a bomb calorimeter
containing 1000 grams of water. The initial temperature of water is 18.98°C. The
specific heat of water is 4.184 J/g °C. The heat capacity of the calorimeter is 615 J/
Som After the reaction the final temperature of the water is 36.38°C.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, roseemariehunter12
When the earth was formed and cooled, why did nickel and iron end up in the center of the earth while basalt and granite ended up in the outer layers
Answers: 3

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 05:40, timmonskids6027
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1
You know the right answer?
CH4+202->CO2+2H20
To begin the experiment, 1.65g of methane CH is burned in a bomb calorimeter
Questions in other subjects:





Mathematics, 03.09.2020 21:01





Biology, 03.09.2020 21:01



