
Chemistry, 01.04.2021 19:20 bryanmcmillianjr
A student titrates an unknown weak acid, HA, to a pale pink phenolphthalein endpoint with 25.0 mL of 0.100 M NaOH. The student then titrates in 11.0 mL of 0.100 M HCl to the solution containing the neutralized unknown acid (HCl was delivered from a second buret). The pH of the resulting solution is pH

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:00, agarcia24101993
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
A student titrates an unknown weak acid, HA, to a pale pink phenolphthalein endpoint with 25.0 mL of...
Questions in other subjects:

Mathematics, 01.04.2020 21:39

Mathematics, 01.04.2020 21:39


Social Studies, 01.04.2020 21:39



Mathematics, 01.04.2020 21:40

Mathematics, 01.04.2020 21:40


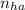 = 2.5 mmoles ------ 1
= 2.5 mmoles ------ 1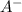 + HCL ---------> HA + C
+ HCL ---------> HA + C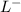
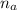 = 1.1 mmoles --------- 2
= 1.1 mmoles --------- 2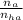 = 1.1 / 2.5 ≈ 1/2 ( this show that when the pH = 4.7 approximately half of the A^- have been converted to HA )
= 1.1 / 2.5 ≈ 1/2 ( this show that when the pH = 4.7 approximately half of the A^- have been converted to HA ) 

