
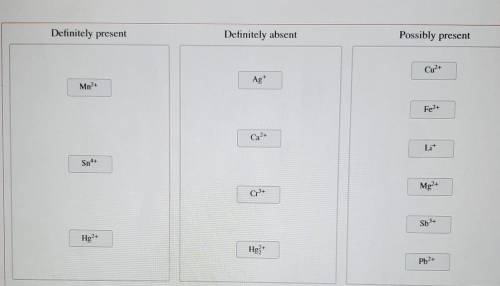
A solution containing a mixture of metal cations was treated with dilute HCI and no precipitate formed. Next, HS was bubbled through the acidic solution. A precipitate formed and was filtered off. Then, the pH was raised to about 8 and HS was again bubbled through the solution. A precipitate again formed and was filtered off. Finally, the solution was treated with a sodium carbonate solution, which resulted in no precipitation.
Classify the metal ions based on whether they were definitely present, definitely absent, or whether it is possible they were present in the original mixture.
Mn2+
Sn4+
Hg2+
Ag+
Ca2+
Cr3+
Hg2 2+
Cu2+
Fe2+
Li+
Mg2+
Sb3+
Pb2+

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, nadikadiaz1
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
A solution containing a mixture of metal cations was treated with dilute HCI and no precipitate form...
Questions in other subjects:


Mathematics, 28.11.2020 06:40




Medicine, 28.11.2020 06:40



History, 28.11.2020 06:40




