ANSWERED
Before you begin, keep in mind these two points:
The timer runs fast, so the m...

ANSWERED
Before you begin, keep in mind these two points:
The timer runs fast, so the minutes go faster than actual minutes.
The temperature will rise during the experiment. If the temperature gets very high, lower it to around 300 K.
Follow these steps, and then record your observations:
Locate the orange reset button on the bottom right side of the screen.
Press reset to start the reaction over.
Drag the top of the ruler upward until it reaches the 40 mark.
Drag the left platform upward until the top of the platform coincides with the 30 mark on the ruler.
Toggle the blue play/pause button to the play position at the bottom of the screen to ensure that the reaction doesn’t start before you’re ready.
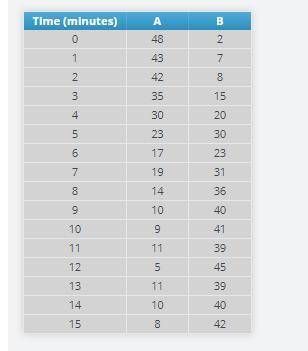
Add 50 A particles, and press the play button on the bottom. Immediately start the timer using the play button on the blue box.
At every minute on the timer, pause the simulation and record the number of A and B particles.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, rex1578
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1


Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
Questions in other subjects:


English, 24.07.2019 05:30

Biology, 24.07.2019 05:30

History, 24.07.2019 05:30




Mathematics, 24.07.2019 05:30




