2 attempts left
Check my work
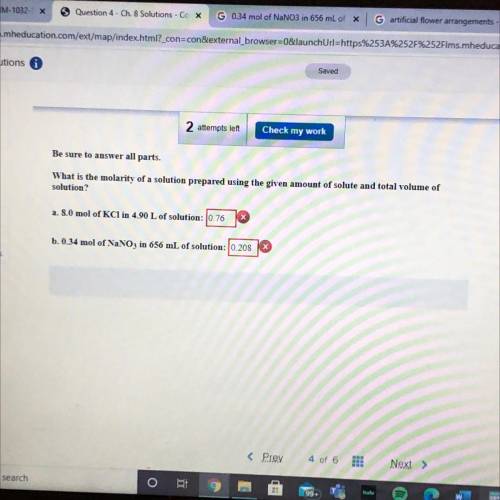
Be sure to answer all parts.
What is the molarity of a so...

Chemistry, 30.03.2021 23:30 princesstn28oqlfir
2 attempts left
Check my work
Be sure to answer all parts.
What is the molarity of a solution prepared using the given amount of solute and total volume of
solution?
a. 8.0 mol of KCl in 4.90 L of solution: 0.76
X
b. 0.34 mol of NaNO3 in 656 mL of solution: 10.208
X

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, dijonmckenzie3
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 08:30, vanessadaniellet21
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1
You know the right answer?
Questions in other subjects:

Computers and Technology, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00


Social Studies, 08.12.2020 01:00

History, 08.12.2020 01:00





