
Chemistry, 30.03.2021 22:00 Lollipop1287
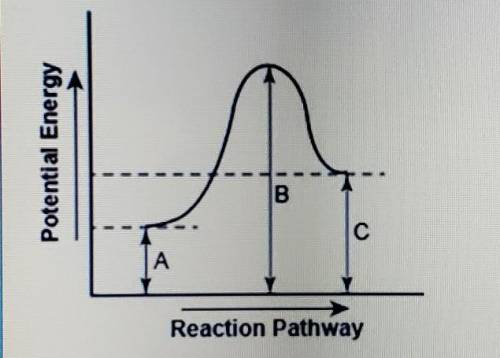
The diagram shows the potential energy changes for a reaction pathway.
part 1: describe how you can determine the total change in enthalpy and activation energy from the diagram, and if each is positive or negative.
part 2: describe how the curve will look if the reaction was exothwrmic. be sure to mention changes in the potential energies of the reactants and products and the sign changes of the enthalpy.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, MrSavannahCat
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1



Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
The diagram shows the potential energy changes for a reaction pathway.
part 1: describe how you can...
Questions in other subjects:


Mathematics, 18.11.2020 04:10

English, 18.11.2020 04:10


Mathematics, 18.11.2020 04:10

History, 18.11.2020 04:10

Mathematics, 18.11.2020 04:10

English, 18.11.2020 04:10


Mathematics, 18.11.2020 04:10



