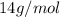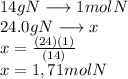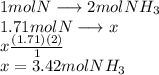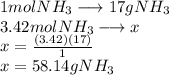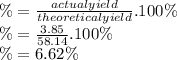Chemistry, 26.08.2019 11:20 ammullims822
A24.0 g sample of nitrogen gas reacts with an excess of hydrogen gas to give an actual yield of 3.85 g nh3. what is the percent yield for this reaction given the reaction: n2(
g. 3h2(
g. --> 2nh3(g

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
A24.0 g sample of nitrogen gas reacts with an excess of hydrogen gas to give an actual yield of 3.85...
Questions in other subjects:

Social Studies, 23.04.2021 14:10


Mathematics, 23.04.2021 14:10

Mathematics, 23.04.2021 14:10



English, 23.04.2021 14:10

Biology, 23.04.2021 14:10

English, 23.04.2021 14:10

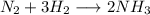
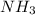 is obtained
is obtained