
Chemistry, 29.03.2021 18:10 yugbug44owwc7w
To increase the temperature of 100.0 g of H2O(s) from -50.0°C to -10.0°C, how much energy is required?
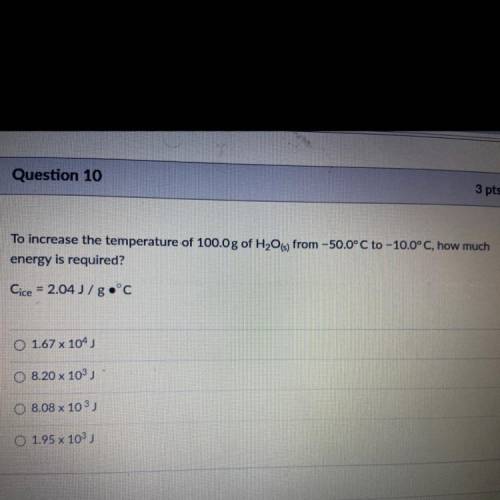

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2



Chemistry, 23.06.2019 13:30, sophie5988
Where are electrons with the lowest energy found? in the nucleus farthest from the nucleus outside the atom closest to the nucleus
Answers: 1
You know the right answer?
To increase the temperature of 100.0 g of H2O(s) from -50.0°C to -10.0°C, how much
energy is requir...
Questions in other subjects:

Mathematics, 30.11.2021 20:00

History, 30.11.2021 20:00




Arts, 30.11.2021 20:00

History, 30.11.2021 20:00


Chemistry, 30.11.2021 20:00

Mathematics, 30.11.2021 20:00



