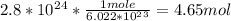How many moles of titanium are in a sample containing 2.8 x 10^24 atoms?
1) 1.69 x 10^48
2) 4...


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, ayahabdulhaqq2
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 21.06.2019 18:00, pressure772
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1
You know the right answer?
Questions in other subjects:




Biology, 24.12.2019 02:31

World Languages, 24.12.2019 02:31



Mathematics, 24.12.2019 02:31

Biology, 24.12.2019 02:31

Arts, 24.12.2019 02:31