
Chemistry, 29.03.2021 02:20 klivingston1012
PLEASE HELP ME YALL I NEED TO GET THIS RIGHT
A chemist wants to extract copper metal from copper chloride solution. The chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (II) chloride. A single replacement reaction takes place. Which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Unbalanced equation: CuCl2 + Al → AlCl3 + Cu (4 points)
a. Approximately 0.36 grams, because copper (II) chloride acts as a limiting reactant
b. Approximately 1.8 grams, because copper (II) chloride acts as a limiting reactant
c. Approximately 0.36 grams, because aluminum acts as a limiting reactant
d. Approximately 1.8 grams, because aluminum acts as a limiting reactant

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, jamccoy3335
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 05:00, pandasarecute53
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 09:00, lrasanaoaksandfurana
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1
You know the right answer?
PLEASE HELP ME YALL I NEED TO GET THIS RIGHT
A chemist wants to extract copper metal from copper ch...
Questions in other subjects:


Social Studies, 25.09.2019 20:30

Chemistry, 25.09.2019 20:30

Social Studies, 25.09.2019 20:30

Social Studies, 25.09.2019 20:30

Social Studies, 25.09.2019 20:30


Social Studies, 25.09.2019 20:30

Social Studies, 25.09.2019 20:30

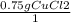 ×
×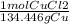 ×
×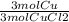 ×
×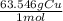 ≈0.35
≈0.35

