
Chemistry, 26.03.2021 22:50 barnhill4755
Which of these equations represent reactions that could be used in constructing an electrochemical cell? Check all that apply.
A. CH4 +2O2 → CO2 + 2H20
B. Cr + Cu^2+ ---> Cr^2+ + Cu
C. 2 Ag+ + Fe → 2Ag + Fe^2+
D. CI^- + Ag^+ → AgCI
E. NH3 +H^+ ---> NH4^4
just got it wrong, the answers are B and C. Just solved my own question
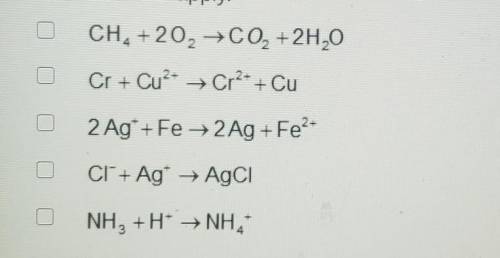

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3


Chemistry, 22.06.2019 22:00, notearslefttocry14
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
Which of these equations represent reactions that could be used in constructing an electrochemical c...
Questions in other subjects:

Mathematics, 12.10.2019 14:30

Mathematics, 12.10.2019 14:30


Physics, 12.10.2019 14:50

Mathematics, 12.10.2019 14:50

Mathematics, 12.10.2019 14:50

Mathematics, 12.10.2019 14:50

Mathematics, 12.10.2019 14:50


Mathematics, 12.10.2019 14:50



