Chemistry, 26.03.2021 21:30 ariellake8551
Nitrogen and water react to form nitrogen monoxide and hydrogen, like this: N2(g) + 2H2O(g) → 2NO(g) +2H2(g)Also, a chemist finds that at a certain temperature the equilibrium mixture of nitrogen, water, nitrogen monoxide, and hydrogen has the following composition: compound pressure at equilibrium N2 0.25 M H20 1.3 M NO 0.33 M H2 1.2 MCalculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
Nitrogen and water react to form nitrogen monoxide and hydrogen, like this: N2(g) + 2H2O(g) → 2NO(g)...
Questions in other subjects:

Physics, 14.12.2019 10:31


Mathematics, 14.12.2019 10:31

Social Studies, 14.12.2019 10:31






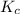
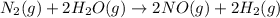
![K_c=\frac{[NO]^2\times [H_2]^2}{[H_2O]^2\times [N_2]^1}](/tpl/images/1224/3364/cca59.png)