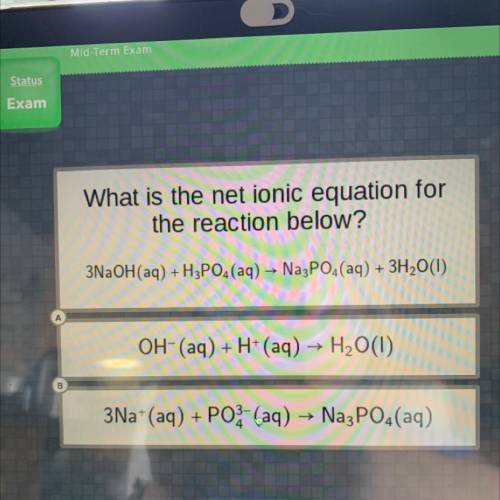
What is the net ionic equation for
the reaction below?
3NaOH(aq) +H3PO4 (aq) → Na3PO4(aq) + 3...

Chemistry, 26.03.2021 20:40 afolmar2006
What is the net ionic equation for
the reaction below?
3NaOH(aq) +H3PO4 (aq) → Na3PO4(aq) + 3H2O(1)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 05.05.2021 21:00


Arts, 05.05.2021 21:00



Mathematics, 05.05.2021 21:00


Mathematics, 05.05.2021 21:00

Biology, 05.05.2021 21:00



