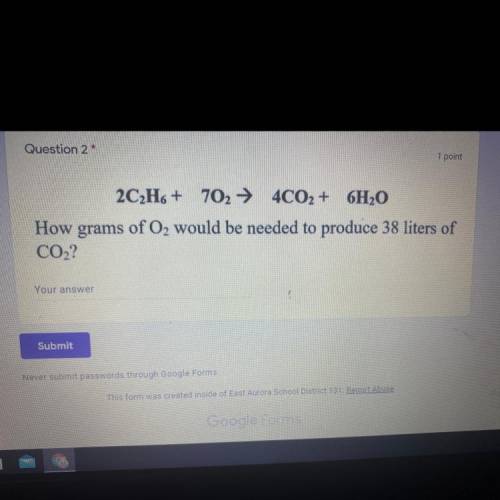
2C2H6 + 702 + 4CO2 + 6H2O
How grams of O2 would be needed to produce 38 liters of
CO2?
<...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
Questions in other subjects:




Mathematics, 06.10.2019 06:10



Chemistry, 06.10.2019 06:10


Mathematics, 06.10.2019 06:10

Mathematics, 06.10.2019 06:10