Chemistry, 26.03.2021 07:10 21hendlill
4NH3 + 502 --> 4NO + 6H20
How much excess reactant is leftover after if 6.30g of ammonia react with
1.80g of oxygen?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, wkalpakchi
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 08:10, 20dyeaubn
Time remaining 58: 10 an atom that has 84 protons and 86 neutrons undergoes a reaction. at the end of the reaction, it has 82 protons and 84 neutrons. what happened to the atom? it accepted radiation in a chemical reaction it donated neutrons to another atom in a chemical reaction it emitted an alpha particle in a nuclear reaction. it accepted protons in a nuclear reaction. mark this and retum save and exit next submit
Answers: 3
You know the right answer?
4NH3 + 502 --> 4NO + 6H20
How much excess reactant is leftover after if 6.30g of ammonia react w...
Questions in other subjects:


History, 08.10.2019 23:50



Mathematics, 09.10.2019 00:00


Biology, 09.10.2019 00:00



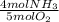 = 0.045 mol NH₃
= 0.045 mol NH₃