Answers: 3


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 05:20, cjking2320
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2

Chemistry, 23.06.2019 17:00, jayjayanyway04
During which of the following phases of the moon do we see the left half of the moon as lit? full moon first quarter moon gibbous moon third quarter moon any is greatly : )
Answers: 1

Chemistry, 23.06.2019 21:30, krystalScott17
Pls why does copper oxide appear on a penny faster when a penny is heated in a flame than when the penny is at room temperature explain
Answers: 1

Chemistry, 23.06.2019 22:30, LukeneedhelpInMath
Write the formula for the conjugate base acid of hexanoic acid
Answers: 1
You know the right answer?
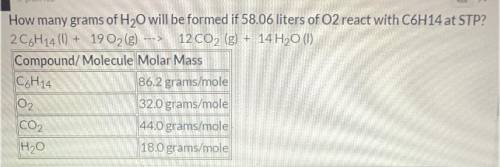
How many grams of H2O will be formed if 58.06 liters of O2 react with C6H14 at STP?
Balanced equati...
Questions in other subjects:

Mathematics, 22.08.2019 13:50

History, 22.08.2019 13:50

Geography, 22.08.2019 13:50




English, 22.08.2019 13:50


Mathematics, 22.08.2019 13:50

Physics, 22.08.2019 13:50