
Chemistry, 26.03.2021 03:50 haileyglowiak8183
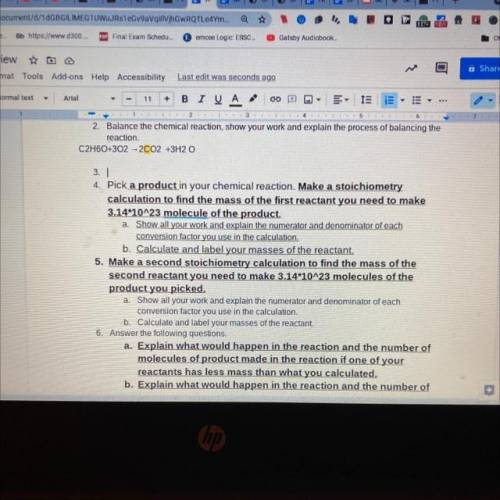
.Pick a product in your chemical reaction. Make a stoichiometry
calculation to find the mass of the first reactant you need to make
3.14*10^23 molecule of the product.
fonch

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 23.06.2019 02:00, jacckiie5176
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 05:00, mprjug6
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
You know the right answer?
.Pick a product in your chemical reaction. Make a stoichiometry
calculation to find the mass of the...
Questions in other subjects:

Mathematics, 23.10.2020 23:20

Mathematics, 23.10.2020 23:20

Mathematics, 23.10.2020 23:20


Mathematics, 23.10.2020 23:20

Mathematics, 23.10.2020 23:20

English, 23.10.2020 23:20

Mathematics, 23.10.2020 23:20





