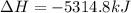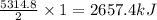Chemistry, 26.03.2021 01:00 turboslayer
Butane C4H10 (g),(Hf = –125.7), combusts in the presence of oxygen to form CO2 (g) (Delta. Hf = –393.5 kJ/mol), and H2O(g) (Delta. Hf = –241.82) in the reaction: 2 upper C subscript 4 upper H subscript 10 (g) plus 13 upper O subscript 2 (g) right arrow 8 upper C upper O subscript 2 plus 10 upper H subscript 2 upper O (g). What is the enthalpy of combustion, per mole, of butane? Use Delta H r x n equals the sum of delta H f of all the products minus the sum of delta H f of all the reactants.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1


Chemistry, 22.06.2019 19:00, Jasoncookies23
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 23.06.2019 00:00, kittenalexis68
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
Butane C4H10 (g),(Hf = –125.7), combusts in the presence of oxygen to form CO2 (g) (Delta. Hf = –393...
Questions in other subjects:


Mathematics, 28.05.2021 21:50

English, 28.05.2021 21:50

Mathematics, 28.05.2021 21:50

Mathematics, 28.05.2021 21:50


History, 28.05.2021 21:50

Chemistry, 28.05.2021 21:50

Arts, 28.05.2021 21:50

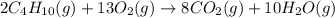
![\Delta H=[n\times H_f_{products}]-[n\times H_f_{reactants}]](/tpl/images/1221/8923/4f68b.png)
![\Delta H=[8\times H_f_{CO_2}+10\times H_f_{H_2O}]-[2\times H_f_{C_4H_{10}+13\times H_f_{O_2}}]](/tpl/images/1221/8923/e94db.png)
![\Delta H=[(8\times -393.5)+(10\times -241.82)]-[(2\times -125.7)+(13\times 0)]](/tpl/images/1221/8923/8343f.png)