The information below describes a redox reaction.
Ag+ (aq) + Al(s) -> Ag(s) + Al^3+ (aq)
<...

Chemistry, 25.03.2021 22:10 iesps010411
The information below describes a redox reaction.
Ag+ (aq) + Al(s) -> Ag(s) + Al^3+ (aq)
Ag+ (aq) + e^- -> Ag(s)
Al(s) -> Al^3+ (aq) + 3e^-
What is the coefficient of silver in the final, balanced equation for this reaction?
A. 1
B. 2
C. 3
D. 4
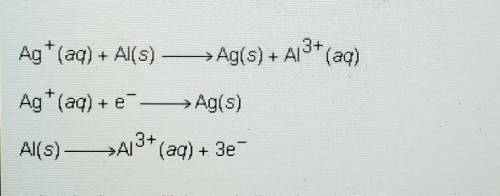

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1



Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
Questions in other subjects:



Mathematics, 24.04.2020 23:22


Mathematics, 24.04.2020 23:22



English, 24.04.2020 23:22




