
Chemistry, 25.03.2021 18:30 Britny2386
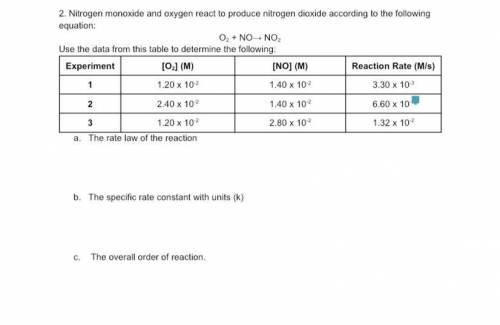
Nitrogen monoxide and oxygen react to produce nitrogen dioxide according to the following equation:
The rate law of the reaction
The specific rate constant with units (k)
The overall order of reaction.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 07:30, libbymcvay
Which statement explains which thermometer is more appropriate to measure the temperature of a liquid at 43.6 degrees celsius a) thermometer a, because it measures temperature more accurately than thermometer b b) thermometer b, because it measures temperature more accurately than thermometer a c) thermometer a, because it measures temperature more precisely than thermometer b d) thermometer b, because it measures temperature more precisely than thermometer a
Answers: 2

Chemistry, 23.06.2019 12:00, Renabelle6350
372 ml is the volume of aluminum, density is 2.70 g/ml what is the mass in grams
Answers: 1
You know the right answer?
Nitrogen monoxide and oxygen react to produce nitrogen dioxide according to the following equation:...
Questions in other subjects:


Social Studies, 02.08.2019 18:00







Social Studies, 02.08.2019 18:10

Mathematics, 02.08.2019 18:10



