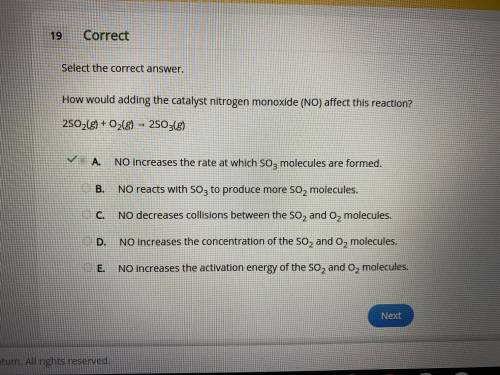
How would adding the catalyst nitrogen monoxide (NO) affect this reaction?
2SO2(g) + O2(g) → 2SO3(g)
A.
NO increases the rate at which SO3 molecules are formed.
B.
NO reacts with SO3 to produce more SO2 molecules.
C.
NO decreases collisions between the SO2 and O2 molecules.
D.
NO increases the concentration of the SO2 and O2 molecules.
E.
NO increases the activation energy of the SO2 and O2 molecules

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, litttyyyu33411
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 23.06.2019 10:30, sbelgirl2000
If a 20.0ml test tube measures 15.0cm, what is the length in meters?
Answers: 1
You know the right answer?
How would adding the catalyst nitrogen monoxide (NO) affect this reaction?
2SO2(g) + O2(g) → 2SO3(g...
Questions in other subjects:


Mathematics, 04.11.2019 09:31







Mathematics, 04.11.2019 09:31