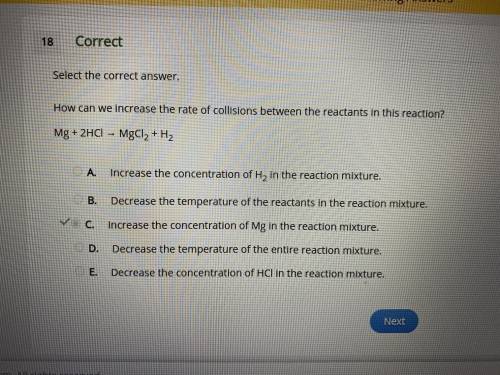
How can we increase the rate of collisions between the reactants in this reaction?
Mg + 2HCl → MgCl2 + H2
A.
Increase the concentration of H2 in the reaction mixture.
B.
Decrease the temperature of the reactants in the reaction mixture.
C.
Increase the concentration of Mg in the reaction mixture.
D.
Decrease the temperature of the entire reaction mixture.
E.
Decrease the concentration of HCl in the reaction mixture.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 11:00, 21villalobosjabez
Which type of fossil does this image depict?
Answers: 1


Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
How can we increase the rate of collisions between the reactants in this reaction?
Mg + 2HCl → MgCl...
Questions in other subjects:


Mathematics, 09.07.2019 18:00

Mathematics, 09.07.2019 18:00


English, 09.07.2019 18:00


Mathematics, 09.07.2019 18:00

Biology, 09.07.2019 18:00