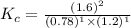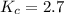Hydrogen and chlorine react to form hydrogen chloride, like this:
H2(g) + Cl,(g) → 2 HCl(g)
...

Chemistry, 24.03.2021 22:30 Kelseygrace8372
Hydrogen and chlorine react to form hydrogen chloride, like this:
H2(g) + Cl,(g) → 2 HCl(g)
Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen, chlorine, and hydrogen chloride has the following composition:
compound pressure at equilibrium
H2 0.78
Cl2 1.2M
HCl 1.6M
Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
Questions in other subjects:

Arts, 19.11.2020 09:20

Mathematics, 19.11.2020 09:20

Mathematics, 19.11.2020 09:20

History, 19.11.2020 09:20

Biology, 19.11.2020 09:20

History, 19.11.2020 09:20

English, 19.11.2020 09:20

Mathematics, 19.11.2020 09:20


Biology, 19.11.2020 09:20

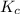
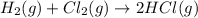
![K_c=\frac{[HCl]^2}{[H_2]^1[I_2]^1}](/tpl/images/1218/2768/5d189.png)