
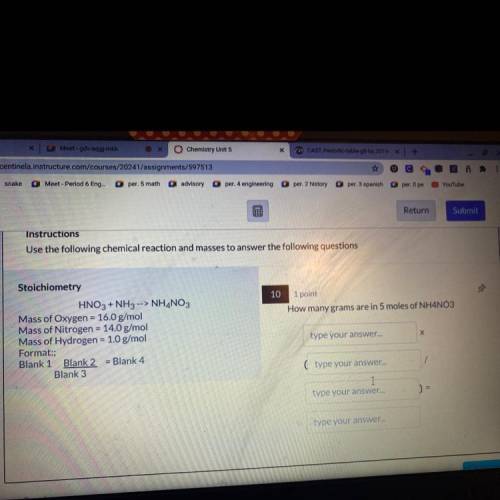
Instructions
Use the following chemical reaction and masses to answer the following questions
10 1 point
How many grams are in 5 moles of NH4NO3
Stoichiometry
HNO3 + NH3 --> NH4NO3
Mass of Oxygen = 16.0 g/mol
Mass of Nitrogen = 14.0 g/mol
Mass of Hydrogen = 1.0 g/mol
Format:;
Blank 1 Blank 2 = Blank 4
Blank 3
type your answer...
Х
(type your answer...
I
type your answer...
type your answer...
PLEASE HELP

Answers: 1


Other questions on the subject: Chemistry



You know the right answer?
Instructions
Use the following chemical reaction and masses to answer the following questions
...
...
Questions in other subjects:

Mathematics, 15.04.2020 03:00

Chemistry, 15.04.2020 03:00










