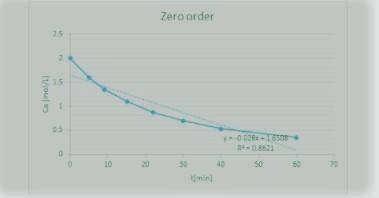
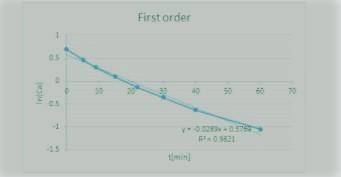
The Reaction
A+ B → C
was carried out in a constant-volume batch reactor where the following concentration measurements were recorded as a function of time:
t min 0 5 9 15 22 30 40 60
CA/mol/L 2 1.6 1.35 1.1 0.87 0.7 0.53 0.35
Required:
Determine the reactor order and the specific rate constant If you were to take more data, where would you place the points

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, NREYESLDS2806
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1


You know the right answer?
The Reaction
A+ B → C
was carried out in a constant-volume batch reactor where the foll...
was carried out in a constant-volume batch reactor where the foll...
Questions in other subjects:

Biology, 27.03.2021 06:00


Chemistry, 27.03.2021 06:00

Mathematics, 27.03.2021 06:00

Mathematics, 27.03.2021 06:00

Mathematics, 27.03.2021 06:00




![\mathbf{[A] = [A_o] -kt ---- (1)}](/tpl/images/1216/9615/f9d14.png)
![\mathbf{In([A])= In ([A_o])-kt ---- (2)}](/tpl/images/1216/9615/6a8a1.png)
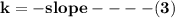
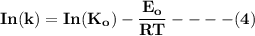
![\mathbf{In([A]) = In ([A_o])- kt}](/tpl/images/1216/9615/2a476.png)